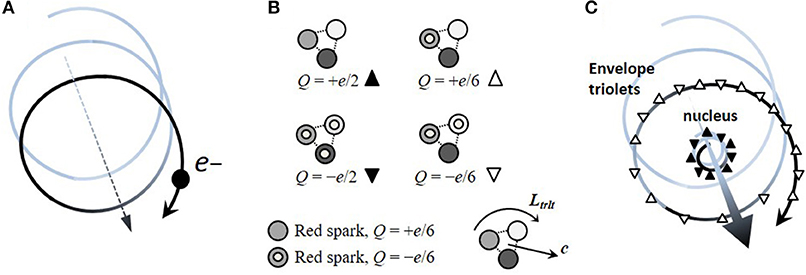
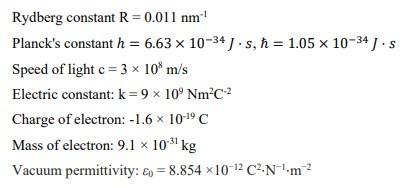Intramolecular Electron-Transfer Rates in Mixed-Valence Triarylamines: Measurement by Variable-Temperature ESR Spectroscopy and Comparison with Optical Data | Journal of the American Chemical Society
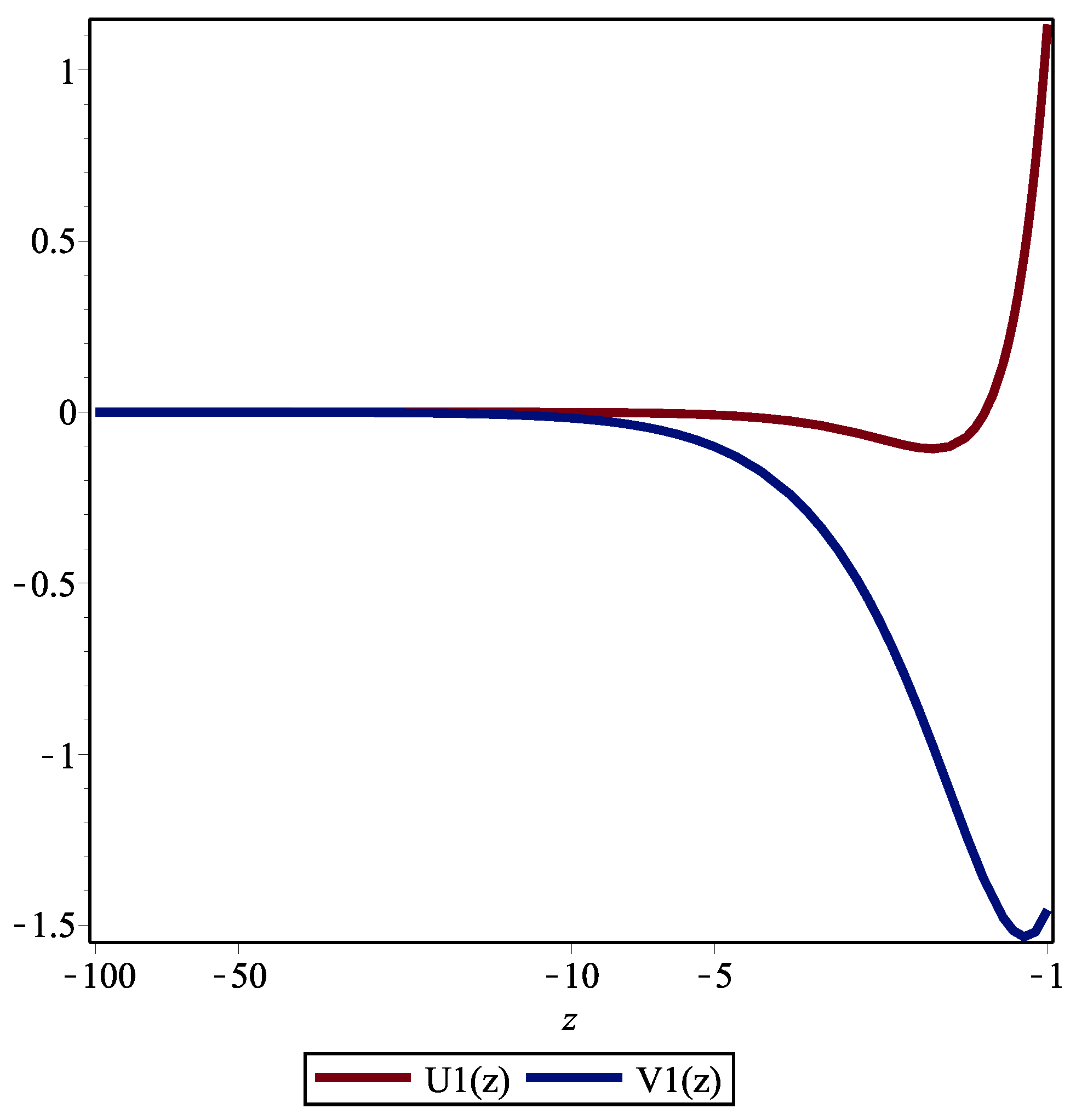
AppliedMath | Free Full-Text | Approximate Nonlocal Symmetries for a Perturbed Schrödinger Equation with a Weak Infinite Power-Law Memory
![Calculate the energy of the light having wavelength 45 nm : [Planck's constant h = 6.63 × 10^-34 Js ; speed of light c = 3 × 10^8 ms^-1 ] Calculate the energy of the light having wavelength 45 nm : [Planck's constant h = 6.63 × 10^-34 Js ; speed of light c = 3 × 10^8 ms^-1 ]](https://dwes9vv9u0550.cloudfront.net/images/8714373/032957da-34c2-47ec-a991-027613566c64.jpg)
Calculate the energy of the light having wavelength 45 nm : [Planck's constant h = 6.63 × 10^-34 Js ; speed of light c = 3 × 10^8 ms^-1 ]

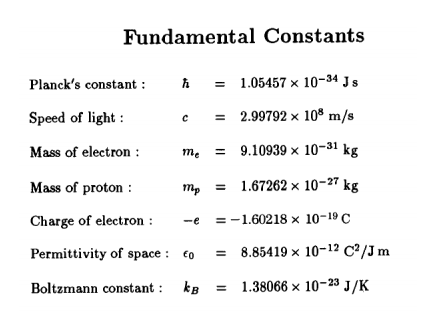
The mass of electron is 9.11 × 10^-31 kg.Planck's constant is 6.626 × 10^-34 Js then the uncertainty involved in the measurement of velocity within a distance of 0.1 A is :
Given: The mass of electron is 9.11 x 10^-31 kg, Planck constant is 6.626 x 10^-34 J s, - Sarthaks eConnect | Largest Online Education Community
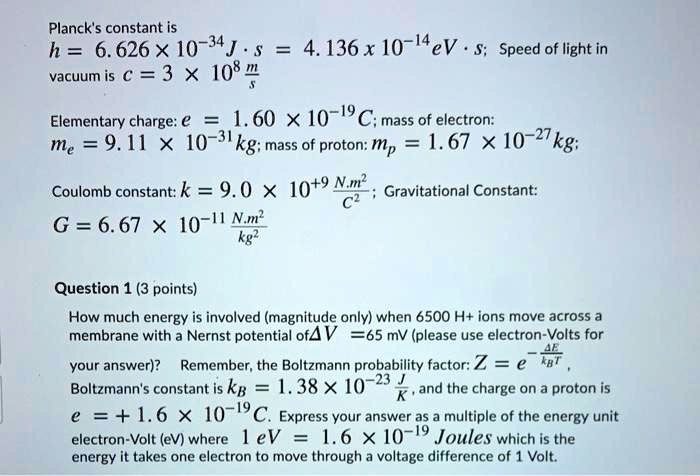
SOLVED: Planck's constant is h = 6.626X 10-34J . vacuum is c =3 X 108@ 4.136 x 10-I4eV . S; Speed of light in Elementary charge: € = 1.60 X1O-19 C; mass
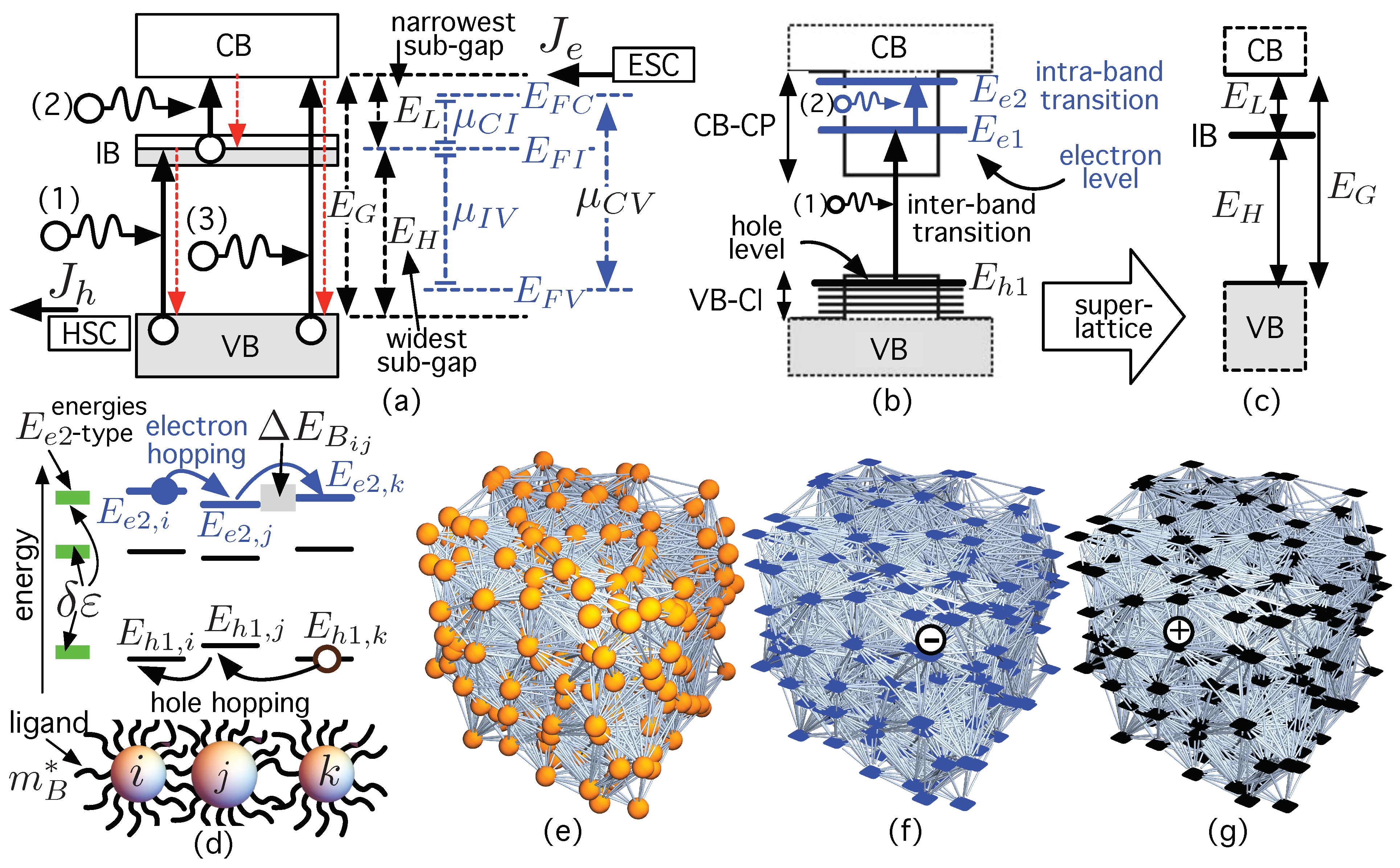
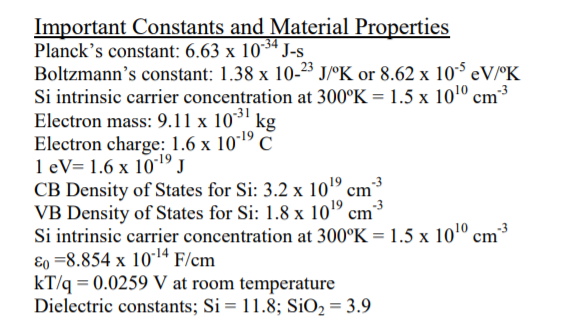
IJMS | Free Full-Text | Carrier Transport in Colloidal Quantum Dot Intermediate Band Solar Cell Materials Using Network Science

Calculate the de - Broglie wavelength of an electron moving with one fifth of the speed of light. Neglect relativistic effects. ( h = 6.63 × 10^-34 J.s., c = 3 × 10^8 m/s , mass of electron = 9 × 10^-31 kg )

Given: The mass of electron is `9.11 � 10^(�31)`Kg Planck constant is `6.626 �10^(�34)` is:- - YouTube