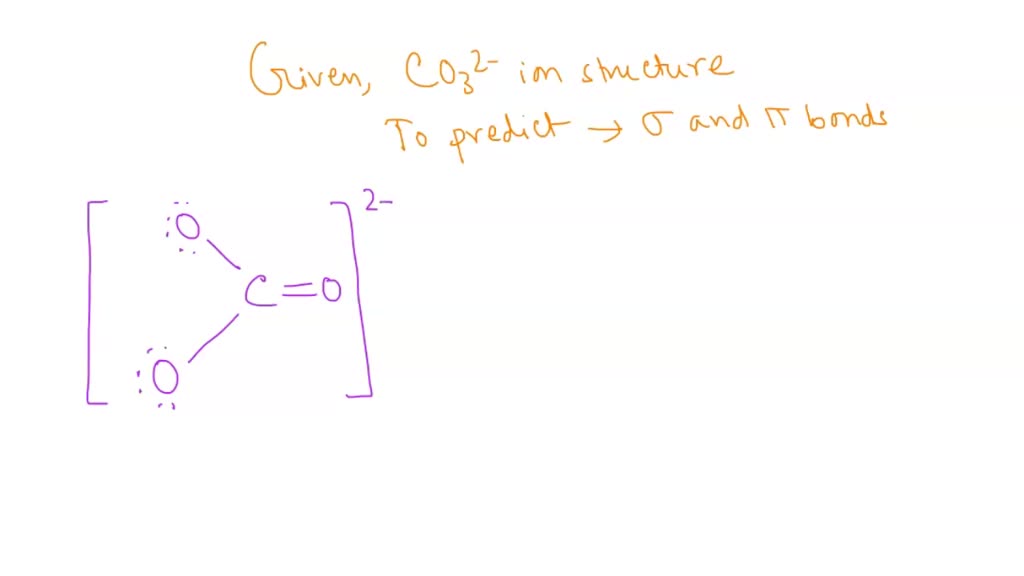
Describe the bonding in the CO32- ion using the LE model. How would the molecular orbital model describe the pi bonding in this species? | Homework.Study.com

Describe the bonding in the CO32- ion using the LE model. How would the molecular orbital model describe the pi bonding in this species? | Homework.Study.com

OneClass: Draw an octet-rule Lewis structure for CO32- State which orbitals or hybrids on C and O ove...
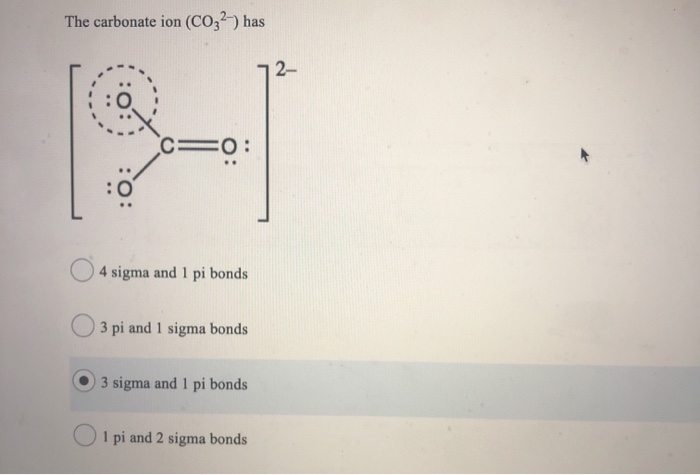
SOLVED: Question 9 (1 point) The carbonate ion (CO32-) has 2- ==0 sigma and pi bonds 3 pi and 1 sigma bonds 3 sigma and pi bonds pi and 2 sigma bonds
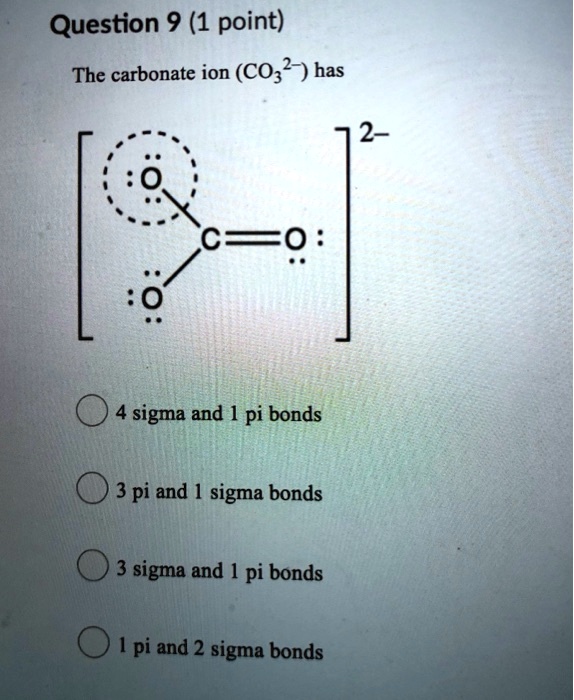
SOLVED: Question 9 (1 point) The carbonate ion (CO32-) has 2- ==0 sigma and pi bonds 3 pi and 1 sigma bonds 3 sigma and pi bonds pi and 2 sigma bonds

Draw two equivalent resonance forms for bicarbonate ion, HCO3-. How many sigma bonds are there? How many pi bonds? | Homework.Study.com

SOLVED: Select the number of sigma and pi bonds in the most favorable structure of the carbonate ion, CO32- one sigma bond, three pi bonds three sigma bonds; one pi bond three







![Q57E Describe the bonding in the CO32... [FREE SOLUTION] | StudySmarter Q57E Describe the bonding in the CO32... [FREE SOLUTION] | StudySmarter](https://studysmarter-mediafiles.s3.amazonaws.com/media/textbook-exercise-images/Q66E-1.jpg?X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Credential=AKIA4OLDUDE42UZHAIET%2F20230525%2Feu-central-1%2Fs3%2Faws4_request&X-Amz-Date=20230525T004937Z&X-Amz-Expires=90000&X-Amz-SignedHeaders=host&X-Amz-Signature=efc08999f6a52a3fd3195cf7da8b3a9f44cccc940d59a402b0367ba381819e17)